Porosome Discovery Promises to Revolutionize Modern Medicine
The discovery of the ‘porosome’, the cell's secretory apparatus, has provided a platform for entry into a new era in drug development and therapy. The porosome has been isolated, its structure and composition determined, and it has been functionally reconstituted into live cells.
Porosome AI: Many diseases including Alzheimer’s and cystic fibrosis, result from secretory defects in the porosome. Undruggable porosome protein targets can now be addressed by incorporating the native functional porosome into diseased cells. Using cryo-EM and AI, the atomic structure of the neuronal porosome is being solved, that has allowed precision-targeting of small molecules to modulate secretory disorder, thereby treating the disease.
PLATFORM


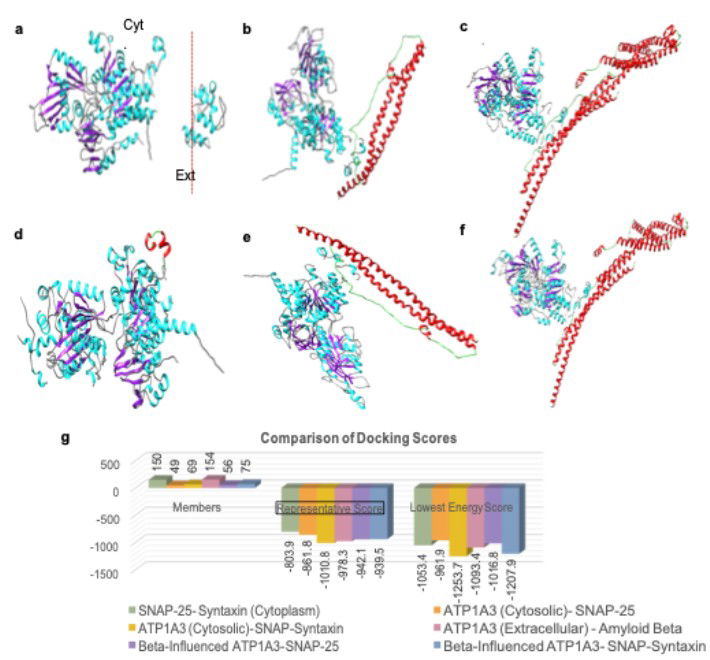
AI reveals binding interactions between porosome proteins SNAP-25, Syntaxin-1, and ATP1A. Amyloid beta binding to the extracellular domain of ATP1A3 negatively impacts the binding interactions between ATP1A3-SNAP-25-Syntaxin-1A in the cytosolic domain within the neuronal porosome complex.
Cellular nanomachines such as the ribosome, the nuclear pore, or the porosome, are protein complexes that have evolved over a billion years to perform life-sustaining cellular functions with great precision and efficiency, yet little is known regarding their assembly from among the millions of proteins in a cell. Understanding the molecular assembly and structure of these nanomachines will provide critical information in the design and development of novel drugs and therapeutic approaches for diseases resulting from their malfunction. Among cellular nanomachines, the cell secretory portal -the ‘porosome’ nanomachine involved in cell-cell communication, for neurotransmission to enable thought, perception, and memory; for the secretion of digestive enzymes to digest food; for release of hormones such as insulin to maintain glucose homeostasis, or the secretion of antibodies for protection from pathogens, is of great scientific and clinical significance.
Our objective is to help elucidate the atomic structure and assembly of the porosome nanomachine to enable new drug development and targeted therapies. Although the porosome is present in all cells, the 15 nm 30-protein neuronal porosome, used for neurotransmitter release and neurotransmission, is the most abundant and well-characterized of porosomes, making it an ideal model system for the proposed study. Recent advances in new and cutting-edge tools and technologies available to Porosome Therapeutics, such as cryo-electron microscopy (cryo-EM) and artificial intelligence (AI) have made it possible for this membrane nanomachine to be explored at the near atomic level, and its assembly determined.
Furthermore, membrane proteins are crucial drug targets, with over 60% of approved drugs controlling cell communication, but historically hard to study due to their hydrophobic nature, requiring detergents for extraction and stabilization. Advances in structural biology, especially cryo-EM and computational methods (AI/ML), are rapidly solving these structures, revealing dynamic states and enabling structure-based drug design for specific targets like GPCRs, ion channels, and transporters, accelerating discovery for diseases from cancer to neurological disorders.
Therefore, the proposed work is transformative, enabling both the molecular-level understanding of the porosome nanomachine and it’s use as a platform for the design and development of new and novel drugs and therapies.
Alzheimer’s Disease: AD is a neurodegenerative disorder characterized by memory loss and personality changes, leading to dementia. It is estimated that in the United States nearly 6.2 million individuals are impacted by AD dementia, and over 50 million globally. AD is increasing at an alarming pace, projected to double by 2050. The primary cause of the cognitive decline that characterizes AD is the loss of neurons, hypothesized as being due to secretory defects in neurotransmitter release and synaptic plasticity. There is growing evidence that in Alzheimer there is impaired neuronal metabolism due to an increase in free radicals and mitochondrial fission, leading to loss in ATP synthesis required for neurotransmission. Therefore, impaired metabolism in neurons may lead to the observed defects in neurotransmitter release, resulting in the loss of neuronal function and connections in regions of the brain involved in memory and reasoning.
Alzheimer's Therapy:
A central challenge in Alzheimer’s disease is understanding the mechanism of neuronal secretory dysfunction. By exploiting AI, we reveal how beta-amyloid disrupts key protein interactions within the neuronal secretory machinery. In a combinatorial strategy of reprogramming the neuronal secretory and metabolic components, we established a dual-target therapeutic framework that repairs synaptic and mitochondrial defects to counteract neurodegeneration in Alzheimer’s. https://www.biorxiv.org/content/10.1101/2025.04.09.647859v1
PRESS RELEASE: https://apnews.com/press-release/business-wire/medical-technology-dementia-alzheimers-disease-medical-research-boston-2975b3c8465f477bbc4331e7590d1253
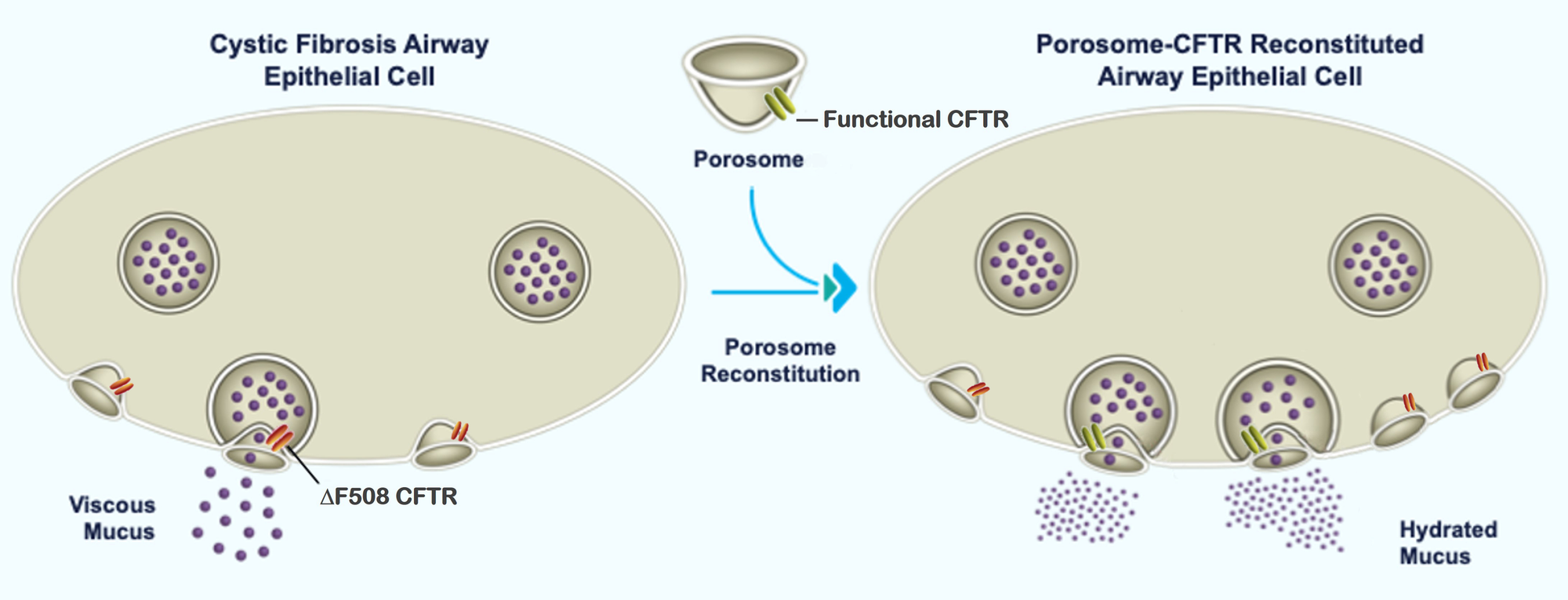
Cystic Fibrosis (CF): The analysis of porosomes in ΔF508-CFTR human CF bronchial epithelial cells shows the loss of several porosome proteins, implying that mutations in CFTR also affect other proteins within the porosome secretory machinery. Consequently, correcting the CFTR protein alone in CF patients is inadequate for overcoming secretory defects in CF patients. In view of this, Porosome Therapeutics has developed proprietary approach to overcome CF by introducing functional mucin-secreting porosomes into cells of patients that have mutated CFTR or in patients without CFTR expression. Porosome reconstitution studies on human bronchial epithelial cells in air-liquid-interface cultures, have demonstrated to be more than twice as effective as the currently available leading CF drug and requires less than 4-fold less frequent administration than the current leading drug. Furthermore, porosome reconstitution studies in animals have demonstrated success, even at the cross-species level, reflecting robust viability and no observable toxicity of the porosome secretory nanomachine’s use as a biologic. [https://www.biorxiv.org/content/10.1101/2024.09.11.612494v1].
OUR CF DRUG RECEIVES ORPHAN DRUG DESIGNATION BY FDA: https://www.businesswire.com/news/home/20250225004448/en/FDA-Grants-Orphan-Drug-Designation-to-Revolutionary-Cystic-Fibrosis-Therapy
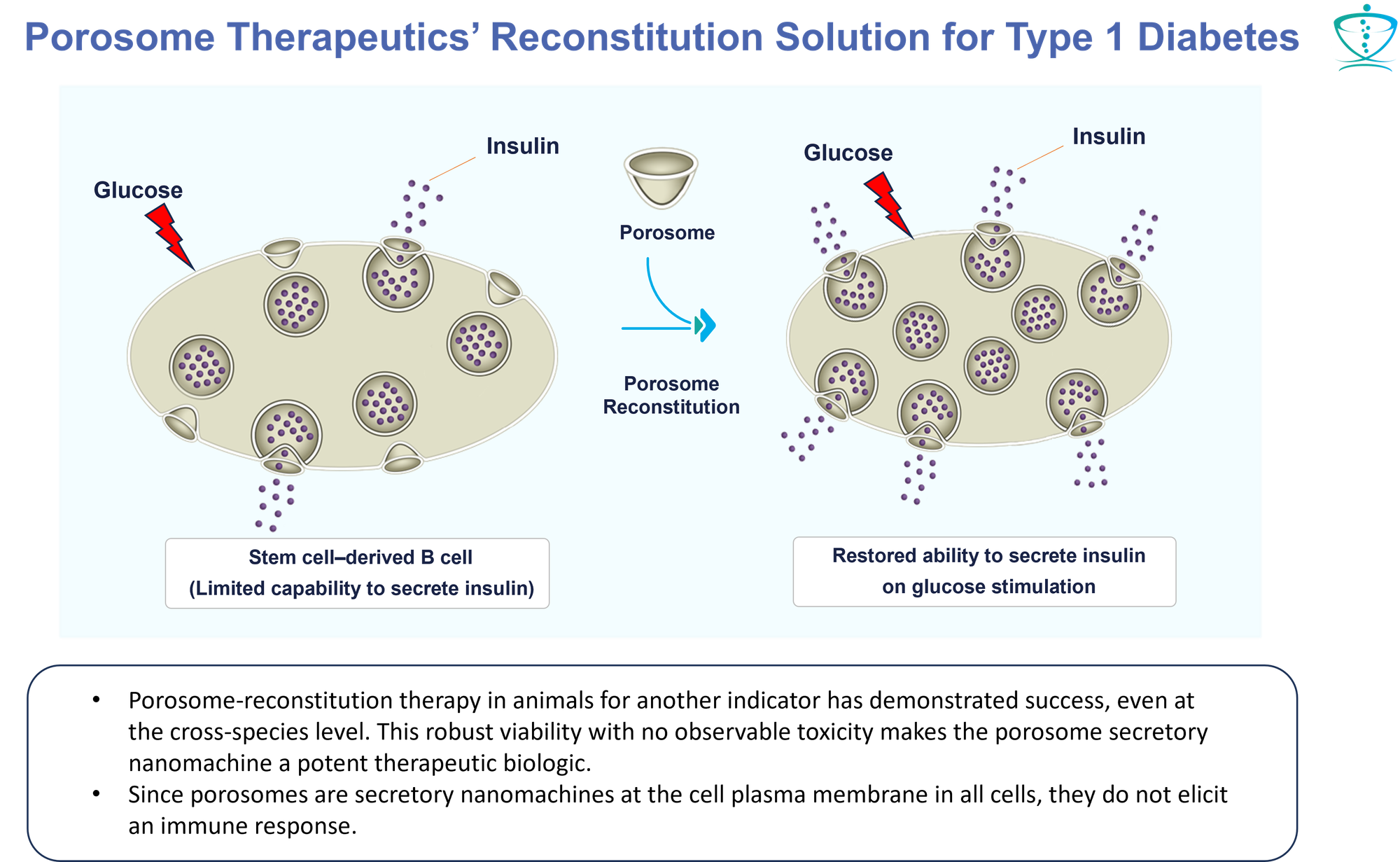
Type 1 Diabetes (T1D)
“SC-Beta cells generated with current directed differentiation protocols are still not quite as functional as primary human b cells, and their transcriptional and chromatin landscape remains immature. Furthermore, the current differentiation methodologies still produce off-target cell types, notably cells that resemble a type of intestinal endocrine called the enterochromaffin cell (EC).” “The ongoing clinical trial led by Vertex Pharmaceuticals has indicated that existing protocols generate SC-islets that are capable of improving glycemic control in human T1D patients. However, further enhancing SC-islets to better mimic the functionality of primary adult islets would reduce the number of cells required for transplantation and make it easier to manufacture sufficient cell numbers. For example, conceptually, if insulin secretion per cell is doubled, then potentially only half as many cells are needed to cure a patient. Reducing graft volume facilitates an easier transplantation procedure, reduces nutrient exchange requirements at the transplantation site, and opens alternative transplantation site possibilities. Furthermore, fewer cells would need to be manufactured, which is significant in terms of both production costs and logistics for a cell-based therapy.” - Hogrebe, N.J., et al. (2023) Development in stem cell-derived islet replacement therapy for treating type 1 diabetes. Cell Stem Cell, https://doi.org/10.1016/j.stem.2023.04.002
Porosome Therapeutics Inc. Approach, Strategy & Solution for iPSC Derived Beta Cell T1D Therapy
Approach: Combining Porosome Therapeutics’ proprietary porosome-based therapeutic approach with induced pluripotent stem cell (iPSC) derived beta cells for T1D replacement therapy.
Strategic Area: iPSC derived Beta cell therapies
Figure 1:
PROBLEM 2 ((c)): It has been observed that not all iPSC reach complete differentiation into functional β cells in vitro and a fraction of the cells may remain undifferentiated, exposing recipients to the risk of teratoma formation post-transplant. Hence, iPSC derived pure β cells are required for transplant (1, please refer to pg 532).
1. Hogrebe, N.J., et al. (2023) Development in stem cell-derived islet replacement therapy for treating type 1 diabetes. Cell Stem Cell, https://doi.org/10.1016/j.stem.2023.04.002
2. Naik, A.R. et al., (2016) Functional reconstitution of the insulin-secreting porosome complex in live cells. Endocrinology, 157:54-60.
3. Porosome Therapeutics Inc. patent
Lung Cancer Bronchorrhea: This is a form of lung adenocarcinoma, in which voluminous watery mucus greater than 100 mL/day is secreted, that could result in respiratory failure. Similarly, in asthma and chronic obstructive pulmonary disease (COPD), airway mucus hypersecretion contributes to impaired mucociliary clearance. Excess mucus production can be targeted via therapies that inhibit mucin synthesis and or mucin secretion in the airways. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors are an example of a targeted therapy to reduce mucus synthesis, as have inhibitors of EGFR's downstream signaling pathways. Their efficacy and safety profiles, however, are currently not acceptable. In contrast, as with the Company’s CF therapy, the reconstitution of porosomes obtained from normal human bronchial epithelial cells into the lung epithelia in patients with Bronchorrhea could restore normal mucin secretion and reverse the disease.
Porosomes: Unveiling Cellular Communication
Life's essence resides within cells, and the average human body has an astounding 38 trillion cells – equal to a linear length that could encircle half the globe. Vitality hinges on cell communication, achieved through the secretion of chemical messages by specialized nanomachines on cell plasma membranes known as "porosomes".
These porosomes, composed of over 30 proteins, play a pivotal role. Research underscores that alterations in specific porosome proteins lead to secretory disorders and diseases like Cystic Fibrosis, Cancer, Diabetes, and neurological conditions. We are actively advancing development of drugs targeting these diseases.
Frontiers of Innovation: Solving the Undruggable Problem
Almost 85% of the human proteome is considered ‘undruggable' [1]. Identifying effective approaches to overcome this limitation and to be able to target these proteins is a major focus in modern medicine. The traditional approach to drug design and development involves the isolation of a specific target; determination of the binding site of the target; and employment of various strategies to design small molecules that will bind to the target site and elicit a therapeutic response.
‘Undruggable’ proteins cannot be targeted by this traditional approach due to a variety of reasons, namely (a) Orthosteric inhibitors may require very high concentrations to outcompete natural ligands resulting in off-target effects and potential toxicity or tolerance issues. (b) Occupation of the binding site results in a complete loss of protein function. (c) The lack of binding pockets on the protein of interest to accomodate a drug. (d) When the targeted goal is to prevent a specific protein-protein interaction, there are often very large interaction surfaces with no obvious binding pockets for a drug. (e) No leads are identified when screening against commercial molecular libraries.
Current approaches to overcoming ‘Undruggable Targets’: (a) Instead of targeting a specific undruggable protein, the selective modulation of an associated protein is used to have the desired effect. This may include depletion via protein degradation or involve protein folding chaperone molecules and/or target amplification. (b) Another approach is increasing the number and variety of molecules available in libraries. (c) Screening against target fragments, rather than larger target molecules which increases the opportunity to identify drug interactions. (d) Fragment screening has also been demonstrated to be effective in targeting protein–protein interactions such as in the human B cell lymphoma 2 (BCL-2) family of regulatory proteins. Fragment-based drug discovery yielded a molecule with nanomolar activity against the target, when traditional library screening failed [2]. (e) Tools such as in silico hotspot mapping based on AI learning from experimentally derived data.
Porosome Therapeutics, Inc. approaches to overcoming the ‘Undruggable Target’ problem: The discovery of the ‘porosome’, the cell's secretory machinery, which is a 30 multi-protein complex, has provided a platform (https://www.porosome.com) for entry into a new era in drug development, design, and therapy. Several of the membrane-associated porosome proteins are ‘undruggable', including the ras family of proteins.
Many diseases, such as cystic fibrosis, Type I and Type II diabetes, neurological disorders, immune disorders and various cancers, result from secretory defects in the porosome. These diseases, including many of those that are due to undruggable porosome protein targets, can now be addressed by incorporating the native functional porosome machinery into diseased cells or by the precision-targeting of small molecules using nanobodies to modulate secretory disorder, thereby treating the disease.
The Company has successfully achieved functional reconstitution of the porosome complex both in a lipid membrane and in live cells, therefore offering significant promise for therapeutic interventions, high-throughput drug screening and the capability to modulate the function of undruggable targets. Similarly, tissue-specific porosome-targeted therapy has the potential to revolutionize medicine by enabling the creation of an unprecedented variety of medicines tailored to a wide spectrum of secretory disorders that result in different diseases. Development of tissue-specific medicines targeted to porosomes not only advances therapeutic precision but also significantly elevates drug safety. Targeted toward the porosome, these medicines aim to effectively mitigate toxicity, minimize associated side effects, and mitigate various risks across the entire spectrum of drugs in treating secretory disorders.
References
1. Targeted protein degradation by PROTACs, T. K. Neklesa et al., Pharmacol Ther., 2017, https://doi.org/10.1016/j.pharmthera.2017.02.027
2. The Challenge of Drugging Undruggable Targets in Cancer: Lessons Learned from Targeting BCL-2 Family Members, G. L. Verdine et al., Clin. Cancer. Res., 2007, https://doi.org/10.1158/1078-0432.CCR-07-2184
By skillfully merging breakthrough discoveries and cutting-edge methodologies, we've embarked on a mission to revolutionize the treatment landscape for these intricate and hitherto elusive health challenges. Our focus is on bringing forth therapies that were once deemed unattainable, opening new avenues of hope for those affected by these conditions.
Revolutionizing Drug Discovery - The Porosome Platform
The landscape of drug discovery has undergone a remarkable transformation propelled by breakthroughs in genomics, gene-editing technologies, stem cell biology, patient-derived organoids, cryo-electron microscopy, synthetic biology, and AI-driven small molecule screening with image analysis. These advancements have redefined the way we approach the development of therapeutics.
However, despite these leaps, a significant challenge persists: approximately eighty-five percent (85%) of disease-causing proteins remain elusive, impervious to current therapeutic interventions. This substantial limitation has spurred the birth of our revolutionary platform, rooted in a pivotal discovery within cell biology – the porosome.
The porosome, a cellular secretory portal essential for intercellular communication, holds the key to overcoming this challenge. Recognized across the industry as a game-changing revelation, our platform shatters the constraints that have hindered progress in drug discovery. By tapping into the potential of the porosome, we are breaking free from the confines that have limited our ability to target critical disease-causing proteins. This marks a new era in drug discovery, one where even the most elusive targets become attainable, fostering hope for transformative therapies that were previously out of reach.
The Porosome Platform is a revolutionary approach that goes beyond the confines of traditional screening methods. With the Porosome Platform, we're equipped to:
- Uncover Crucial Targets: Venturing beyond conventional limits, we delve into the realm of functional protein and lipid targets involved in cell communication through secretion processes.
- Precision Target proteins that cause disease: Our capabilities extend to the realm of pinpoint precision, enabling us to identify small molecules that can intricately bind to even the most complex targets, including those traditionally deemed "undruggable".
- Unveil New Modalities for treatment of disease: The Porosome platform opens doors to the modulation of secretory functions, ushering in new possibilities to address challenges that have persisted for ages. This includes tackling conditions such as cancer, diabetes, neurological disorders, and other historically elusive health issues.
Pathway to Discovery through the Porosome Platform
Within our pursuit, we continually screen thousands of small molecules across the 30+ proteins in the porosome complex, notably within neurons and the endocrine and exocrine pancreas. This process identifies novel high-affinity pockets within target proteins of interest. Data from this screening informs the creation of innovative covalent small molecules precisely engaging crucial drug targets.
The effectiveness of these compounds, either alone or in synergistic formulations, is first assessed in silico by gauging their binding affinities to target porosome proteins and lipids. This is followed by analyses involving cell cultures, organoids, and animal studies, paving the way for clinical trials. Driven by our proprietary industrial-scale platform, Porosome Therapeutics redefines druggability, crafting medications pertinent to a spectrum of secretory disorders.
The platform consists of five interconnected components, each contributing to its potential for therapeutic applications and drug screening.
- Functional reconstitution of the porosome complex which has been achieved both in a lipid membrane (as demonstrated in a study published in Biophysical Journal in 2003;85(3):2035-43) and in live cells (as shown in research published in Endocrinology in 2016;157(1):54-60). These breakthroughs offer significant promise for therapeutic interventions and high-throughput drug screening.
- Tissue-specific porosome-targeted therapy which has the potential to revolutionize medicine development by enabling the creation of an unprecedented variety of medicines tailored to a wide spectrum of medical conditions.
- Development of tissue-specific medicines targeted to porosomes not only advances therapeutic precision but also significantly elevates drug safety. By concentrating on the porosome, these medicines aim to effectively mitigate toxicity, minimize associated side effects, and mitigate various risks across the entirety of our drug portfolio.
- The integration of nanobody-assisted drug delivery technology further enhances the platform's capabilities. This technology, highlighted in a publication in Micron in 2017;92:25-31, accelerates the creation of precision medicines for diverse medical indications. This innovation paves the way for breakthroughs in drug design and delivery.
- The platform is supported by a global collaborative research network that capitalizes on a wealth of intellectual resources. This network enables the acceleration of drug design and development by leveraging collective expertise, thereby enhancing accuracy, speed, and efficiency in the research process.

In addition to these core components, the platform also addresses the challenge of diseases caused by pathogen entry into cells through the plasma membrane. Researchers are actively engaged in the identification of small molecule drugs that regulate various transport functions at the cell membrane. This strategic approach aims to prevent the entry of pathogenic viruses and mitigate associated diseases.
Cystic Fibrosis
Today, the average life expectancy for people with CF who live to adulthood, is about 44 years (https://medlineplus.gov/ency/article/000107.htm).
Cystic Fibrosis arises from a protein dysfunction stemming from mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). This chloride channel governs mucus viscosity, notably in lung epithelial cells. While current treatments mainly address the malfunctioning CFTR protein, Porosome Therapeutics pioneers an alternative. By reintroducing a functional CFTR-associated porosome secretory complex into lung epithelial cells affected by CF, we aim to rectify the deficiency caused by the non-functional mutated CFTR.
This innovative approach offers a comprehensive, curative solution for CF treatment. It has the potential to substantially reduce morbidity, mortality, and enhance the quality of life for CF patients. Moreover, this novel approach could significantly decrease the need for lung transplants among CF patients.
Concurrently, Porosome Therapeutics’ complementary small molecule therapy, distinct from existing treatments, targets porosome-associated proteins interacting with the CFTR protein. Leveraging our proprietary precision drug targeting approach employing small molecule-associated nanobodies, this strategy holds promise for heightened effectiveness and minimal side effects. Depending on the state of the CF disease, both reconstitution and small molecule therapies can be applied.
Type 1 and Type 2 Diabetes
Porosome Therapeutics has developed an innovative proprietary approach to effectively address the insulin secretion defects in both Type 1 and Type 2 Diabetes. This solution-set centers on the beta cells (β-cells) responsible for insulin production within the disease context:
- Utilizing Fundamental Sub-Cellular Mechanisms: For the first time, we harness the pivotal sub-cellular secretory nanomachine directly relevant to diabetes—the porosome complex. This complex operates in conjunction with the water-transporting aquaporin (AQP) water channel, crucial for regulated insulin secretion from β-cells.
- Deploying Paradigm-Shifting Therapeutic Technology: Our revolutionary approach introduces a proprietary therapeutic technology that fundamentally "corrects" the manifestations of Type 1 Diabetes. We accomplish this by "incorporating" functional porosome machinery into pancreatic β-cells derived from stem cells. This groundbreaking step holds promise for a comprehensive and long-lasting correction of diabetes-related deficiencies.
- Porosome-Targeted Small Molecule Therapies: Our strategy extends to providing targeted small molecule therapies. These therapies are designed to restore the insulin-secreting and insulin-producing capabilities of diseased β-cells, effectively returning them to a state of functional normalcy.
- Collaborative Research on Human Islets: In tandem with leading investigators in the field, we conduct joint research focused on human islets. This collaborative effort allows us to tap into diverse expertise, enhancing the precision and impact of our approach.
Lung Adenocarcinoma
Lung cancer stands as the foremost contributor to both mortality and morbidity on a global scale. Its predominant manifestation takes the form of adenocarcinomas. Notably, diverse lung cancers display deficiencies in secretory processes within lung epithelial cells. A prime illustration is mucinous adenocarcinoma, where the inability to properly secrete mucus results in the constriction of airways. This mirrors symptoms reminiscent of those found in cystic fibrosis (CF), including obstructive pneumonia due to the failure of efficient mucus drainage.
In response to this pressing challenge, Porosome Therapeutics has devised a comprehensive strategy. This multifaceted approach entails the utilization of small molecule drugs and nanobodies, both directed at the porosome proteome within lung epithelial cells. The primary aim of this strategy is to intervene in and enhance the intricate process of mucus secretion. By doing so, the goal is to potentially alleviate obstructive pneumonia and other distressing symptoms associated with lung cancer.
Diseases associated with defects in neuronal porosome proteins
Alzheimer's disease presents distinct alterations in neuronal porosome proteins, with noticeable shifts in protein levels. Specifically, levels of CNPase (2,3-cyclic nucleotide phosphodiesterase) and heat shock protein 70 (HSP70) increase, while dihydropyrimidinase-related protein-2 (DRP-2) levels decrease. Notably, within the neurons of Alzheimer's patients, there are significant reductions in the porosome proteins SNAP-25 and synaptophysin.
CNPase levels consistently decrease in the frontal and temporal cortex of individuals with Alzheimer's disease and Down's syndrome. Likewise, reduced CNPase levels are observed in the anterior frontal cortex of individuals with schizophrenia. Additionally, a genetic allele associated with lowered CNPase levels is linked to schizophrenia, implying a genetic factor at play.
Conversely, abnormal levels of SNAP-25 exhibit distinct correlations. Elevated SNAP-25 levels are linked to cognitive deficits, potentially contributing to impaired cognitive function. In contrast, the absence of SNAP-25 is associated with Huntington’s disease, emphasizing its role in neurodegenerative disorders.
The intricate interplay of these porosome proteins underscores their significance in the pathophysiology of diverse neurological disorders, shedding light on potential avenues for therapeutic interventions and genetic influences.
Summary
Porosome Therapeutics’ Porosome Platform harnesses the intricate mechanisms of cellular communication for revolutionary drug discovery. It is unlocking the potential of previously overlooked proteins and offering innovative solutions for a wide spectrum of health challenges.
 |  |
| CNPase (2,3-cyclic nucleotide phosphodiesterase) | DRP-2 (Dihydropyrimidinase-related protein-2) |
Contact
- One Boston Place, Boston, MA 02108, United States
- Ste 2603
- contact@Porosome.com
- 9am-6pm